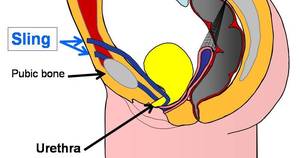
A bladder sling is utilized to deal with stress urinary incontinence (SUI) in women. A bladder sling is made from strips of medical mesh and is normally inserted through one vaginal incision and 2 little abdominal lacerations.
SUI, which primarily happens in women, is the spontaneous loss of urine caused by exercises, such as laughing and sneezing. Specialists state that’s since the urethra– the tube that carries urine out of the body– is much shorter in women (2 inches) than men (10 inches). In addition, the pressures of pregnancy and giving birth, and even aging, can contribute to SUI.
When successful, a bladder sling treatment can alter a patient’s life. All frequently, though, unpleasant issues have made patients wish they had actually not chosen to remedy SUI with this type of surgery.
To create a bladder sling, a specialist utilizes strips of tissue or a synthetic material like mesh to create a pelvic hammock around the bladder neck and urethra to keep them closed throughout regular activities.
Bladder sling procedures are frequently puzzled with surgical treatments performed to correct pelvic organ prolapse (POP), a comparable condition that can be treated by transvaginal mesh. The surgeries, although they involve a lot of the same organs, are different.
There are several kinds of bladder slings authorized by the U.S. Food and Drug Administration (FDA). Some of the most typical consist of:
- Tension-free vaginal tape (TVT) sling: This outpatient treatment uses a polypropylene mesh tape, which functions as a sling under the urethra and is kept in place by the patient’s own tissue rather than stitches. This debuted in 1996.
- Transobturator tape (TOT or TVT-O) sling: This tension-free surgery has less danger of bladder and bowel injury in comparison with the TVT sling since there is no have to blindly pass a big needle through the retropubic area when inserting the mesh tape. It was invented in France and gave the United States in 2002.
- Mini-sling: This treatment has less danger of problems relative to the previous two due to the fact that it gets rid of the need for abdominal or groin incisions. In this treatment, a little single vaginal laceration is made and a mesh tape is placed in a U-shaped setup in the mid-urethra. It is the cutting edge and was released in 2006.
One research study of 75 women with a median age of 47 discovered a general complication rate of 21.5 percent. Complications consisted of one bladder injury, one patient with extreme bleeding and one vaginal extrusion. In general, the research study concluded that TVT “appears exceptional” to TOT in performance one year removed from surgery however that “TOT appears more secure.”
Severe Complications of Bladder Sling Surgery
Problems from bladder slings made from surgical mesh hurt and cause both physical and psychological issues, and corrections typically require multiple treatments and revision surgeries.
Some severe problems after bladder-sling surgery have actually been reported. Patients who reported post-surgery problems said they have lengthened problem urinating or they incur brand-new symptoms of incontinence, specifically urgency. The risks associated with the surgery likewise consist of infections at screw or staple points, internal bleeding or injury to an organ.
The most major issues, however, have been reported with artificial slings that have actually worn down into close-by organs in the pelvic cavity, such as the vagina, and rectum. Organ perforation has actually also been reported. This happens when the sling pierces an organ. The patient might also experience swelling, which suggests the body has actually rejected the sling.
Mesh contraction is another serious complication. It can result in severe pelvic pain and unpleasant sexual relations.
After each surgery, patients must be prepared to substantially alter their lives during the recovery period. They ought to expect that they will not have the ability to urinate by themselves for numerous weeks and need to be comfortable using a catheter, a little tube they can insert themselves. There likewise will be pain that can be controlled with prescription medication from their medical professionals, and over the counter fiber options are recommended for constipation.
Patients will not be allowed to carry out any heavy lifting for a minimum of one month and in some cases approximately 3 months after the treatment. To reduce the risk of infection, patients need to give up sexual relations or making use of the bathtub, pool or hot tubs until the stitches dissolve, which can use up to two months.
According to one New England Journal of Medicine (NEJM) research that compared bladder slings to the older Burch strategy, the most typical side effect was urinary system infections, which took place in 63 percent of women going through a sling procedure.
Issues with ObTape
ObTape, a bladder sling made by Mentor Corporation, had a multitude of problems. Mentor stopped making ObTape in 2006.
Issues with ObTape, which was implanted in women from 2003 to 2006, are twofold. First, its safety record was connected back to a product that the FDA had currently recalled– Boston Scientific’s Protegen sling, which was removed the marketplace in 1999.
Second, ObTape was authorized due to the fact that it apparently matched other tapes utilized in bladder slings, but, in fact, it was made from more dense products that caused bladder sling complications. The thicker weave did not enable the tissues around it to receive nutrients and the body could not easily accept the device.
ObTape was authorized by the FDA under its 510(k) process, which implies it did not need to go through patient screening since the FDA agreed it was similar to other items on the marketplace.
Meanwhile, Mentor offered its medical urology company to Coloplast and after that was obtained by Johnson & Johnson.
Hundreds File Bladder Sling Lawsuits
Numerous claims that have actually been filed because 2008 over the defective Mentor ObTape have actually been funneled into a Multidistrict Litigation (MDL) in the United States District Court for the Middle District of Georgia. The MDL, presided over by Judge Clay D. Land, allows the pre-trial procedures of similar cases to be consolidated. The MDL is slowly progressing, and of June 2015, over 400 cases are still pending in federal court. Earlier in 2015, J&J provided to settle about 100 cases for a concealed amount.
About 35,000 women in the United States have had a sling implant that used ObTape, but just a portion of patients have stepped forward up until now to look for option.