While no macular degeneration treatment currently accepted for usage in the United States is most likely to totally recover vision lost to the eye illness, some drugs – such as Lucentis – might have the ability to slow or prevent extra vision loss and even enhance continuing to be vision to some level.
Likewise, specific investigational treatments have actually shown pledge for reversing a minimum of some vision loss in people affected by age-related macular degeneration (AMD).
FDA-Approved Macular Degeneration Treatments
Implantable telescope. In July 2010, the FDA authorized a small, implantable device that multiplies images onto the retina to enhance central vision harmed by AMD or Stargardt’s macular dystrophy.
The Implantable Miniature Telescope (IMT) from VisionCare Ophthalmic Technologies amplifies the general image while decreasing the relative size of the central blind spot caused by macular degeneration.
About the size of a pea, the gadget is for certain individuals who experience severe vision loss triggered by advanced (“end-stage”) macular degeneration.
“The scientific arise from the essential FDA trial have actually shown we can put this small telescope prosthesis inside the eye to help patients see better and, for some, even to levels at which they can recognize people and facial expressions that they might not before,” said Kathryn A. Colby, MD, PhD, ophthalmic cosmetic surgeon at Massachusetts Eye and Ear Infirmary in Boston and an assistant professor of ophthalmology at Harvard Medical School.
In June 2012, VisionCare revealed the IMT implant became available across the country as part of its CentraSight treatment program for individuals with end-stage AMD.
Likewise, the treatment is covered by Medicare for eligible patients who have central blindness in both eyes that can not be fixed with eyeglasses, contact lenses, medication or other eye surgery, the company states.
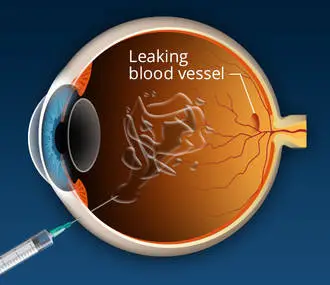
Lucentis. Accepted by the FDA in June 2006 for dealing with the advanced or “wet” kind of macular degeneration, Lucentis (ranibizumab) is a type of the colorectal cancer treatment drug, Avastin.
Lucentis works by hindering proteins called vascular endothelial development element (VEGF), which stimulate the growth of new members vessels in the body. VEGF is believed to add to development of macular degeneration by promoting the growth of unusual blood vessels in the retina.
Genentech, in partnership with Novartis Ophthalmics, markets the drug.
Lucentis scientific trial results causing FDA approval were quite positive, specifically when compared with outcomes of previously authorized treatments. In late 2005, Genentech revealed results of one research study showing improved or steady vision in about 95 percent of individuals, compared to just about 60 percent of individuals getting another approved treatment.
Vision enhancement with Lucentis was significant. While only 11 percent of the control group might see 20/40 or much better following the study, about 40 percent of Lucentis patients had the ability to do so. Overall, about one-third of patients going through Lucentis treatment in FDA scientific trials experienced vision enhancement.
Comparable results with Lucentis treatment continue to be reported in more recent researches.
Lucentis is administered through regular monthly injections into the eye. An FDA news release about the approval said unusual negative responses to the drug primarily were related to the injection itself. Problems of Lucentis treatment can include swelling within the eye (endophthalmitis), increased eye pressure, distressing cataract and detached retina.
The above-mentioned cancer drug Avastin is substantially less expensive and appears to produce similar results in macular degeneration treatment, though it is not FDA-approved for this usage.
The Lucentis vs. Avastin argument continues while an ongoing scientific trial compares outcomes and safety of the two treatments. The first-year outcomes of the Comparison of AMD Treatments Trials (CATT) were revealed in May 2011, with efficiency considered about equal however long-term safety not yet compared.
Macugen
This treatment for AMD makes use of a therapeutic particle to attack VEGF in the eye. Macugen, developed by Eyetech Inc. and Pfizer, is administered through injections into the eye, with treatments needed every six weeks.
In clinical trials, 33 percent of patients getting Macugen preserved or enhanced their vision compared to only 22 percent in the control group. Macugen likewise assisted slow the rate of vision loss for many age-related macular degeneration patients.
Fewer than 1 percent of patients receiving Macugen experienced severe side effects such as a separated retina or endophthalmitis. Less significant side effects, such as eye floaters and discomfort, took place in approximately 40 percent of patients.
Eylea
Like Lucentis and Macugen, Eylea is designed to prevent the action of VEGF in damp (neovascular) AMD. It got FDA approval for this purpose in November 2011.
A possible advantage of Eylea, likewise referred to as VEGF Trap-Eye, is that its recommended dose is an injection into the eye every eight weeks (after 3 preliminary monthly injections), rather than every four weeks like Lucentis.
Eylea was developed by Regeneron Pharmaceuticals and Bayer HealthCare. Regeneron reported that pre-approval clinical trials showed advantages and side effects that were similar to those of Lucentis.
2 studies published in American Journal of Ophthalmology in July 2013 showed that eyes with persistent wet AMD that were resistant to several injections of Lucentis or Avastin (or both) showed stable visual acuity and reduced intensity of AMD appearance with less regular injections of Eylea.
Based upon these findings, the study authors concluded Eylea is an effective alternative to Lucentis and Avastin for the treatment of neovascular AMD, with the added advantage of less often required injections.
Visudyne drug treatment (Photodynamic Therapy or PDT)
Visudyne was the very first drug treatment accepted for treatment of the damp kind of macular degeneration. It is just for those patients who have new blood vessel development (neovascularization) under the retina in a well defined, distinct pattern called “mainly classic.” About 40 to 60 percent of brand-new damp AMD patients have this type of the illness, according to Novartis, the company that markets Visudyne.
In this treatment procedure, the doctor injects Visudyne into your arm, then turns on the drug as it travels through the retinal capillary by shining a low-energy laser beam into your eye. Visudyne is turned on by the laser light, which produces a chemical reaction that destroys unusual capillary. The treatment is virtually painless, according to Novartis.
One in six Visudyne patients reveals improved vision, or about twice as many patients as those who do not undergo Visudyne treatment. Recent studies likewise show significant slowing down of AMD development in lots of patients getting Visudyne drug treatment.
In one research study, 225 eyes with damp AMD were treated with Visudyne, and 114 comparable eyes were not. After 24 months, legal loss of sight happened in 28 percent of the Visudyne group and in 45 percent of the non-treated group.
Visudyne sometimes is utilized in addition to Lucentis or Avastin as a treatment for wet macular degeneration. Other light-activated drugs developed to carry out in methods similar to Visudyne are currently in advancement.
Laser treatment
Laser photocoagulation is another macular degeneration treatment for wet AMD. The treatment utilizes laser light to damage or seal new members vessels to prevent leakage.
A significant drawback of laser photocoagulation, however, is that it produces lots of little retinal scars, which trigger blind spots in the patient’s visual field. For this reason, the laser procedure is no longer commonly used to treat AMD. Scientists are dealing with ways to minimize the scarring and also are studying laser treatments for dry macular degeneration, however progress has actually been slow.
In laser photocoagulation treatment of damp AMD, a laser is utilized to destroy and seal new members vessels to avoid leak and more damage to the retina.
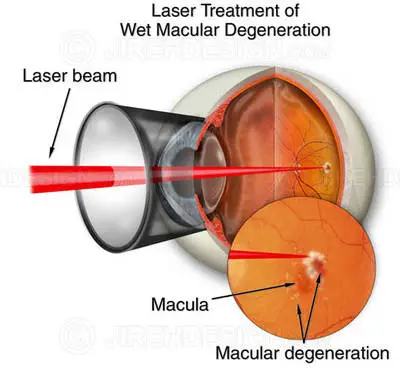
Only about 15 to 20 percent of patients with the damp kind of AMD have the kind of bleeding under the retina (choroidal neovascularization or CNV) that would qualify them for this kind of treatment.
And recently, treatments such as Visudyne photodynamic treatment have actually ended up being more popular than laser photocoagulation for the treatment of damp macular degeneration with CNV.
In the future, investigational macular degeneration treatments that are not yet FDA-approved may supply even better results than those that are currently readily available.